An oxalic acid solution contains the following species in varying concentrations: H2C2O4, HC2O4-, C2O4^2-, and H+. Which of the above four species can act only as acids, which can act Chemistry. Write equations for the dissociation of the following in water. Do not include states in your answer.So a base based on some other mechanism, such as NH3 (which does not contain OH− ions as part of its formula), will be a weak base. The nitrogen in C5H5N would act as a proton acceptor and therefore can be considered a base, but because it does not contain an The reaction is as followsbisulfate ion HSO4- can act as either an acid or a base in water solution. Which of the following is the equilibrium constant expression for the dissociation of the weak acid HOCl?The bases that partially dissociate after dissolution in water are generally described as Weak Bases. This means that it does not ionize entirely when in The molecules will dissociate but the ions will combine to provide an acid molecule. The weak acid provides a lesser amount of H+ ions resulting in...Which of the following ions will act as a weak base in water? OH- in water is always a strong base. This should be immediately thrown out considering this question. You must consider what actually would happen with each of these reactions in water
Strong and Weak Acids and Bases and Their Salts - Introductory...
Which of the following ions will act as a weak base in water? Dissociation of Weak acids and Bases, Polyprotic Acids & Hydrolysis of Salts 10) What is the concentration of H' ions in a 0.010M solution of HCN?Q. The following statements regarding Baron Bunny is false. Baron Bunny's explosion delay is based on it's charge time. Amber's Precise Shot Talent allows her to gain additional CRIT Rate for a certain period after hitting an opponents Weak Spot. Q. Which of the following Book Collections was written...Chemistry - Acid Base Equilibria. University. Pontifical and Royal University of Santo Tomas, The Catholic University of the Philippines.Which of the following ions will act as a weak base in water?

CHAPTER 14 Acids and Bases
What is the pH of a 0.15 M aqueous solution of sodium formate (NaHCO2)? Chapter 16 Question 42 - Multiple-Choice:Part A Which of the following ions will act as a weak base in water?none of these will act as a weak base in water. OH- is normally a strong base but with an amphoteric metal such as zinc or aluminium it can be a weak base or a weak acid. Cl- normally neutral In HCl it is the H+ which is the acid.Many substances behave as weak bases in water. Such substances react with water PRACTICE EXERCISE. Which of the following compounds should produce the highest pH as a 0.05 M solution: pyridine, methylamine, or nitrous acid? Consequently, the ClO- ion acts as a weak base in waterHow do you determine which of the following is the weakest base? Also the shape of the azide molecule is linear and it provides this molecule a better approach, hence it acts as a better nucleophile as well Thus the -OH ions are weak bases as compared to the azide ions.What is the net ionic equation for the acid-base reaction that occurs when acetic acid and potassium hydroxide solutions are mixed? Which of the following statements is FALSE given the following net ionic equation? (a) If all the water evaporated away, the salt remaining could possibly be CuS.
OH- is usually a strong base however with an amphoteric metal such as zinc or aluminium it may be a weak base or a weak acid.
Cl- usually impartial In HCl it is the H+ which is the acid
NO3- in most cases impartial In HNO3 it is the H+ which is acidic.
ClO- A weak acid it's salts with alkalis will display a weak base because of hydrolysis, yielding hypochlorous acid and OH- ions.
Chapter 12 - Acid-Base Chemistry

Solved: Which Of The Following Ions Will Act As A Weak Bas ...

Preparation of Amines - Chemistry LibreTexts

Relative strengths of select conjugate aci... | Clutch Prep

Chemistry Partner

Types of acids bases and salts. 10 Common Acids and ...

Which of the following ions will act as a weak base in ...

Chemistry Archive | June 18, 2017 | Chegg.com
Using the data in the table which of the conjugate bases ...

3. Classify the following as either acidic, basic or ...

Bronsted Acids and Bases

Acid Base Neutralization Reactions & Net Ionic Equations ...

Solved: Which Of The Following Ions Will Act As A Weak Bas ...
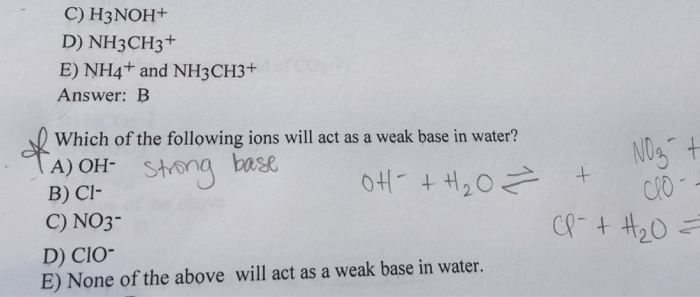
Brønsted-Lowry Acids and Bases - Chemistry

Solved: Designing And Preparing A Buffer REPORT SHEETS ONL ...

PPT - Chapter 16: Acids and Bases, A Molecular Look ...

PPT - Chapter 15: Aqueous Equilibria PowerPoint ...

Chemistry Archive | October 11, 2017 | Chegg.com
Lewis acids and bases

PPT - Chapter 16: Acids and Bases, A Molecular Look ...

Question 4 (1 point) Which type of alcohol is most easily ...







No comments:
Post a Comment